How to meet IRB requirements
By Aaron Small●6 min. read●Oct 10, 2024
When planning a research study that involves human subjects, getting approval from an oversight committee is essential. But the process can feel like you’re walking in the dark without a headlamp.
This guide helps you traverse the tricky path ahead and reach your research goals faster.
We’ll walk you through:
Tips when interfacing with an IRB (Institutional Review Board)
Preparing your research proposal for IRB approval
Understanding incentive structures and effective payout methods
Grab your safety goggles – let’s dive in.
Step 1: Research design and development
Say you want to investigate the effects of musical podcasts for children, or want to test your new sleep apnea machine before it hits the market.
Time to get writing.
Getting all your documents in order is certainly the biggest hurdle to overcome. The IRB expects a detailed research proposal including:
A concise review of the literature
Study objectives
Research questions & methodology
Participant recruitment
Incentive structure and details
Informed consent
Data collection and storage procedures
It’s a lot. Know your plan, and lay it all out.
Cole and Montgomery share a few tips for this stage.
Be specific about participants. Make it clear who you want as part of your study. Will it be faculty? College students? People under the age of eighteen? Different rules apply for different ages.
Say more. Give your IRB enough information so they’re comfortable with all aspects of your project.
Establish a point of contact with your IRB. Avoid bottlenecks by engaging with one specific person there.

Step 2: The IRB application
Forms, forms, and more forms.
Your IRB expects a fairly complete and detailed research protocol. Include all the ingredients, and don’t skip any step that’s critical to your study.
Set a deadline and chip away one chunk at a time. But before you hit send, hit share.
“Peer review before you file your application,” says Holly Cole.
Your colleagues may catch any potential errors and can help you get unstuck. You could try throwing them an incentive, like coffee or lunch, to get their eye.
Step 3: Pre-review and revisions
Time for your soft review. The IRB specialist (who you hopefully now know by name) reviews your application to see that it meets the requirements. If they find any issues, they’ll notify you. Then you address them and fire off the application.
Step 4: IRB review
The IRB takes out the red pen and officially evaluates your application. Reviews are placed into three categories, ranging from simple to complex (Liberale & Kovach, 2017):
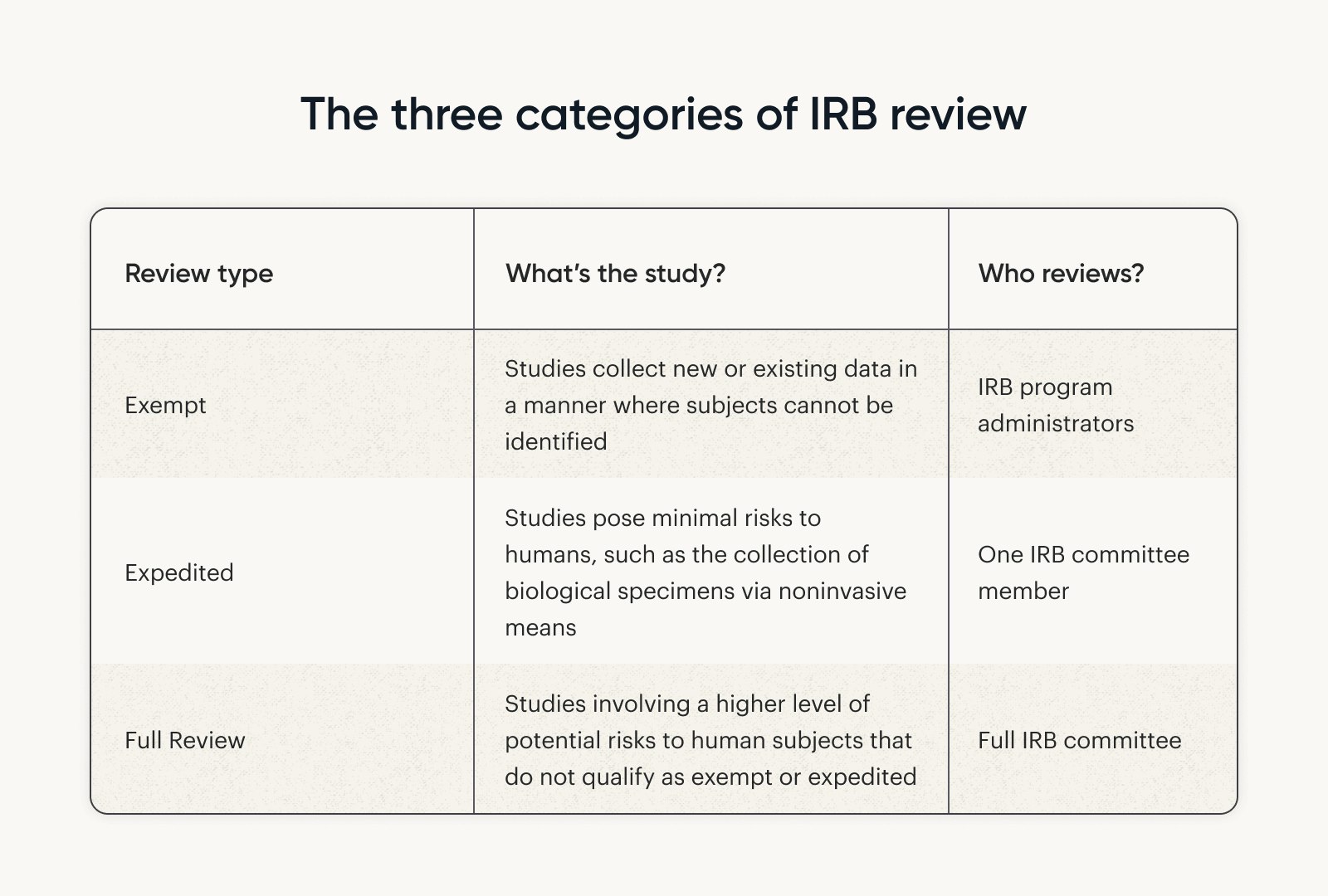
Which type of review do you need?
Make the right request and save time and effort. For example, if your research involves “minimal risk” to participants, you likely qualify for an expedited review.
Step 5: Revisions
The IRB provides feedback, you address their comments, and submit revised documents.
Feedback may include:
Requests for clarification
Modifications to the study protocol
Additional safeguards for participant protection.
Step 6: IRB approval
Pop the bubbly or grab a smoothie. Your application is finally, finally approved by the IRB, and you’re allowed to proceed with your study.
If this is your first research application, congrats on seeing it through and getting it done. Note: this approval could be conditional, requiring progress reports submitted throughout the study.
But the hard part’s done. Now it’s time to get people to participate.
Give rewards, get the results
Money might not be the only motivation for volunteers (Stunkel & Grady, 2011), but offering monetary incentives to encourage participation is a common practice in research.
You want people in your study, and people want some incentives.
Incentives can range from small payments to larger sums in the form of gift cards or other tangible benefits. Singer & Bossarte and Robinson et al. and more studies indicate that larger upfront cash incentives are more effective for recruitment and retention.
Getting people to participate is pivotal for academic research and clinical trials, especially in longitudinal studies where drop-offs can destroy data quality.
And low enrollment in your study would mean it’s game over before you begin – a real waste of resources and no scientific benefit (Kittarman et al. (2011).
What’s the IRB stance on incentives?
US federal guidelines touch on the ethical considerations of offering incentives but provide limited specific guidance for IRBs on the topic.
As a result, IRBs often take a more conservative approach to payment (Largent & Lynch, 2017) to minimize the possibility of undue influence on participants (45 CFR 46.116).
Singer, E., & Bossarte, R. M. (2006) flat out reject the idea that incentives are coercive – because there’s no harm or threat on the table.
Largent & Lynch also push back against this notion of coercion, in their paper: Paying research participants: The outsized influence of “undue influence.”
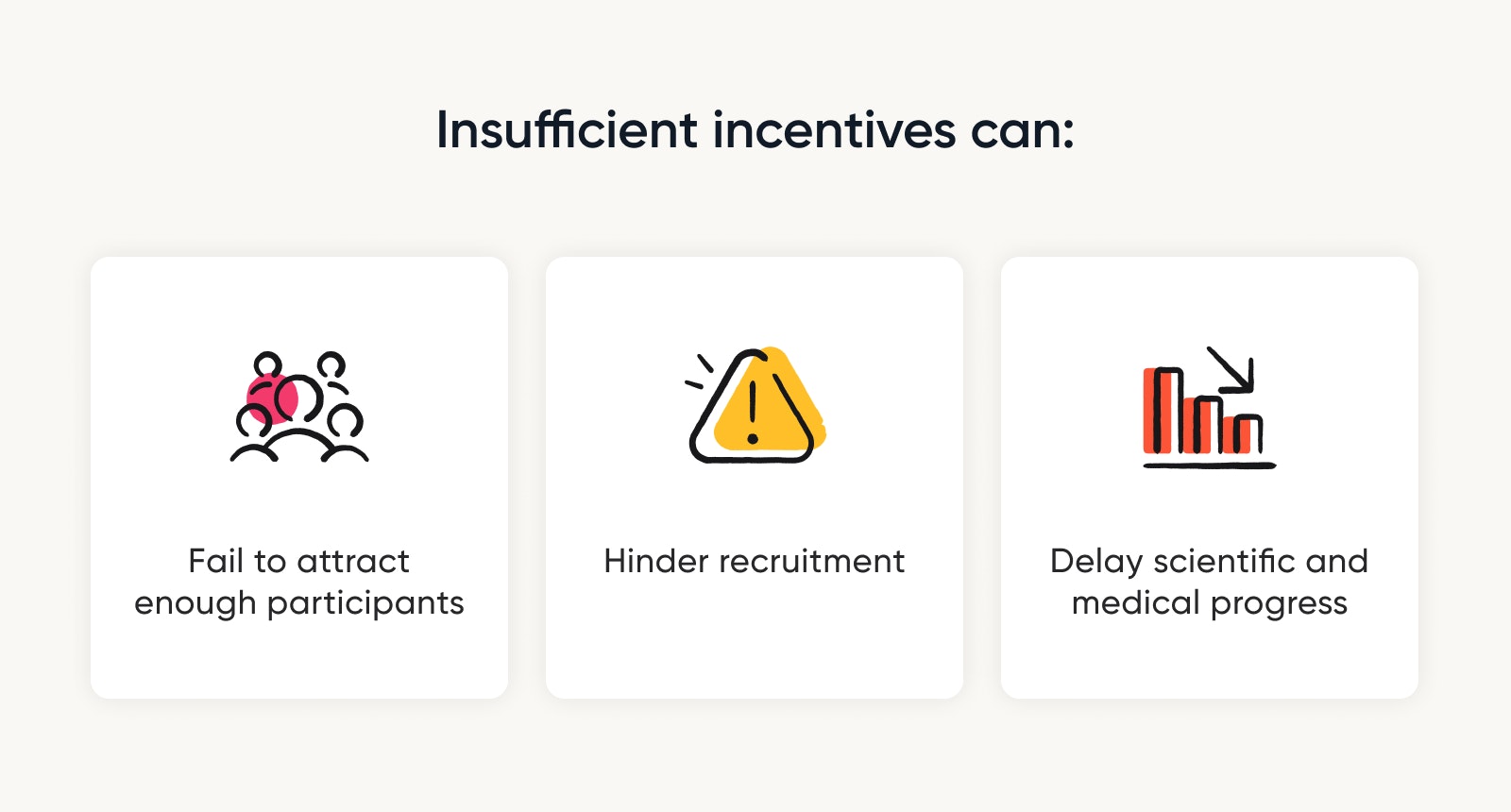
The authors believe that taking a conservative approach to incentives:
Burdens participants and fails to attract a diverse set of subjects
Hinders recruitment and delays scientific and medical progress
They raise the counterargument — rather than assuming payments are too high, IRBs should start asking whether payments are high enough.
Because they work to motivate people and get them in the door. And studies show they also improve retention for participant follow-ups.
Still, each IRB may set specific rules and regulations around incentives. Contact them directly to find out what’s fair game and what’s out of bounds.
Want to see some real-world examples? Here are guidelines from the University of Michigan and U of Oregon.
In both cases, things like taxes and tracking reward totals get more complicated when incentives are higher than $100.
And yet, you can reduce perceived risks and ensure swifter IRB approval with the following best practices.
Advice when planning your incentive structure
Tip 1: Be clear
Make it known that the incentive is offered for participation purposes only. Provide a clear rationale for the amount of the incentive.
Use simple and non-technical language. Help participants understand the study's purpose, what is expected of them, and make a more informed decision (Nusbaum et al., 2017).
Tip 2: Document the payout process
Your consent form should include a separate section for incentives that lists: the total incentive amount and rollout schedule.
Are there conditions where someone would receive partial or no payment?
Spell out the payment method or platform used (e.g., cash, gift card, debit card) and timeline for payment. Will it be given immediately or months after the study?
Cole stresses timing. ��“Are you going to pay participants seven days after the study? Thirty days?” Impress your IRB and lay it all out.
Tip 3: Know your target population (and protect their PII)
One key to successfully incentivizing research is to understand what people find appealing and getting it to them in a way that’s fast, simple, and doesn’t put them at risk.
When it comes to rewards, “be age appropriate,” Montgomery said. “Think of the audience.”
A Target or Starbucks gift card is a smart and safe bet to attract moms. In-game currency to the multiplayer game League of Legends? Maybe not so much.
Collect only the necessary info required to reduce the risk of potential privacy breaches. Store that data in password-protected folders to be destroyed later.
Pro tip: Tremendous lets you choose from over 1,000 retailers, allowing your participants how to spend the reward.
More common mistakes to avoid when getting IRB approval for incentives
Confusing language. Forms must be written at an appropriate reading level for the target audience. All recruitment materials, scripts, and protocols must be submitted: (email, website postings, flyers, advertisements, verbal scripts).
Incorrect terms. Be sure to use the right terms (state or IRB guidelines) for incentives in recruitment materials.
Unreasonable rewards. Justify the amount of incentives you offer relative to similar studies. Provide a clear rationale for the incentive amount to strengthen your case.
No privacy protection. If payment methods other than cash are used, researchers should detail how payment and personal information will be processed securely.
How to get started with Tremendous
Our team would be more than happy to show you how Tremendous works. You’ll be surprised at how intuitive and easy it is to use. You can start sending your first payout in five minutes or less.
From uploading your recipient list to choosing the reward types you want to offer, it’s very straightforward.
Book a demo and schedule a short call with our product experts.
We’ll discuss:
Your business goals for sending payouts
How Tremendous can saves you time, money, and hassle
Making Tremendous fit your needs, however simple or complex
We’ve partnered with institutions big and small, and love a challenge. Contact us today and let’s get your research program off the ground. (Well, at least the incentives part. We don’t own any lab coats.)
Updated October 10, 2024